Upper Arm Blood Pressure Monitor 24h BP Recorder+Software+Finger Pulse Oximeter
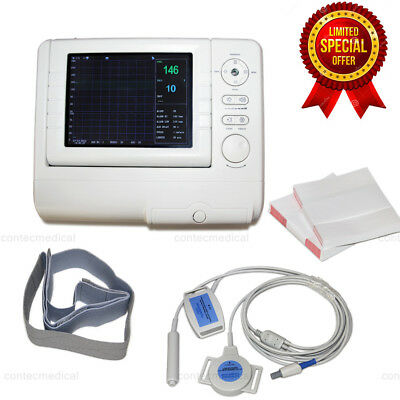
CMS800G Fetal Monitor Ultrasound Baby Heart Rate Fetal Move Marker TOCO Sensor
Description
Instructions
Fetal Monitor can acquire fetal heart rate, maternal uterine contraction when pregnancies over 28 weeks to provide reference data for clinical use.The monitor can be used individually or connected with PC through RJ45 Interface for the purpose of central monitoring.
It is only suitable for the equipment in hospitals, clinics, doctors offices and patients at home by professional medical personnel.
Major Features
◆Light dexterous appearance, tops horizontally and walls can be hoisted
◆8.0 “screen color LCD display, rotatable screen to 60°
◆Display of the patient data and curve clearly
◆FHR 120 BPM~160 BPM normal range label
◆Manual records fetal movement
◆Sound and color alarm for high and low fetal heart rate
◆Continuous 24-hour real-time monitoring function
◆Continuous 12-hour patient curve and data storage , playback and print
◆With picture freeze function
◆Optional English interface
◆Single, Twins Monitoring optional
◆9 crystal board band pulsed wave transducer
◆Extra-long life, high-resolution built-in thermal recorder
◆Built-in communication port, can be connected with central monitoring system.
Main performance
Security:
Anti-shock types: Facilities I, no internal power supply Anti-electric Shock Degree: B Working Voltage: AC 100 V~240 V Frequency: 50 Hz/60 Hz P<60 VA Fuse: T1.6AL250VDisplay
Dimensions: 8.0 “color LCD display, folding 60 degree
Display
Content: bed No. , pregnancy weeks, age, paper speed, date, time , volume, alarm status, transducer connection status, recorder status, FHR data and wave, Contraction data and wave, Fetal move times and mark etc.
Print:
Record Paper two-double type Z
Print Width: 112 mm
Valid Print Width: 104 mm
Paper output speed: 1 cm/min、2 cm/min、3 cm/min(optional)
Data Precision: ±5 %(X Roll),±1 %(Y Roll)
Record Content: hospital , bed No. ,name, pregnancy weeks, patient No., paper speed, date, time , FHR data and wave, Contraction data and wave, Fetal move times and mark etc.
Ultrasound probe:
Nominal Frequency: 1.0 MHz
Work Frequency: 1.0 MHz±10 %
Negative peak sound pressure : p_<1 MPa
Output beam intensity : Iob<20 mW/cm2
The peak time space peak intensity: Ispta<100 mW/cm2
The average time space peak intensity: Isata<10 mW/cm2
FHR Rang: 50 BPM~240 BPM
Resolution: 1 BPM
Accuracy : ±2 BPM
TOCO
TOCO range: 0~100 %
Resolution: 1 %
Nonlinear error: <±10 %
RZ way: Manually
Fetal Marking
For the manual button (the operation of pregnant women), there will be a mark display in the bottom area of FHR wave display section.
FHR Alarm:
Alarm for high and low FHR, which exceeds appointed limit.
Accessories
Sell in standard:
◆Transducers(Ultrasound TransducerⅠ, TOCO Transducer, Remote Marker)
◆Abdomen belt
◆Record Paper
◆Power supply line
◆Earth line
◆Two Fuses
◆User manual
Sell in addition
◆Ultrasound TransducerⅡ
Physical Characteristics
Size: 320 mm (length) × 260 mm (width) × 80 mm (height)
Weight: about 3 kg
Shipping
1. We will ship the item within 1 working day after receiving your payment,
ship from China warehouse by China Post 1-4 weeks Arrival.
2. All items will be shipped to buy’s ebay address. Please check your ebay address is right.
3.The item will be declared as lower value for shipping only.We’d like to cooperate with you and declare the value of it as you want us to,so that it is easier for you to clear the custom.
International Buyers – Please Note:
* Import duties, taxes and charges are not included in the item price or shipping charges. These charges are the buyer’s responsibility.
* Please check with your country customs office to determine what additional costs will be prior to bidding
Terms of Sale
Buy safe products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
About Us
CONTEC Medical System was founded in 1992 as a professional medical equipment producer.
At present,there are more than 1200 employees in our company .Our product lines cover a wide range of 13 categories,and have been distributed over 30 countries.
We are the manufacture of medical equipment for more than 20 years, main products include ECG machine,
Patient monitor,EEG machine,pulse oximeter,Fetal doppler,Blood pressure monitor and so on..
Contact Us
Looking forward to establishing a successful business relationship with you.
Contact Person:Vivian Zhang
Only English User manual,Please contact us if you need other language version.